I've been sick since last weekend. It's mostly a head cold but it tried really hard to get into my lungs, I was able to fight it off and am feeling better today, but that's not the story.
Three nights ago it hit me the worst, I wanted, no I needed to sleep, but Arden's BG was high and being very stubborn so I wasn't able to until about 2:30 am. On a day when I wasn't sick I'd take 2:30 with a smile, but like I said, I wasn't feeling good and so it was tough. But I survived. With about five hours of sleep under my belt, the next day drug on. I was a bit of a wreck if I'm being honest and my cold was doing it's best to advance into my chest. I needed sleep badly now, so I guess you can imagine what happened.
Arden got the cold too, now I knew why her BGs were so stubborn. She stayed home from school and we were sick together. But that night, that second night of my three night tale of woe, it was rough. BGs fought me like a bull and I wasn't able to lay my head down for good until 4:38 am, but I still had to get up at 7:15 the next morning. Now my ass was officially dragging. Every time I tried to sit down yesterday my eyes would close so I stayed busy doing household chores. When the evening came Arden was feeling much better, she didn't get it as badly as I did thankfully and I was hopeful about her BGs and my chances for some overdue rest.
Before bed last night Arden's BG was a respectable 123 on her CGM, 140 with a finger stick and I was smelling sleep. I stayed up for another two hours to make sure she wasn't going to drop unexpectedly and sometime around 12:30 am I set a temp basal restricting her overnight basal rate because I knew I was going to fall to sleep and stay that way. It makes me nauseous to admit this, but I was happy to trade a waking 190 for seven hours of sleep.
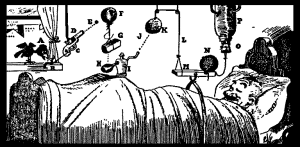
This is what I woke up to.

Now I know what the FDA will say to my next statement and I don't care. We need something to help us. After a while you stop hearing the beeps and feeling the vibrations. Other days you may just be too tired or sick to react. For all that continuous glucose monitoring brings to the daytime, it just doesn't cut it after we lay our heads to rest.
Arden's DexCom receiver, your DexCom receiver should come with a dock, maybe it's an alarm clock too. I should be able to buy a companion clock for my room, for our living room, there should be an app. F&%king alarms and whistles and lights should blare in my ears and shine in my face. Hell I'd wear a bracelet that shocked me when Arden's BGs fell too low. Give me a Rube Goldberg that bashes me in the face, give us something... anything.
Now I am quite sure that the manufacturers of CGMs understand how much an alert system of some kind is necessary and this isn't the first time that I or others have ranted on the subject, but I am tired of hearing that, "the FDA won't allow that". I don't give a damn that each piece of tech that you add to a process makes getting clearance nearly impossible, just someone get the nerve to try. Walk defiantly forward and try to break new ground. Please.
Do it before I die of exhaustion, do it before Arden dies in her sleep. Do it before it decimates another life, another family. Be bold so we can not just live, but live better. Look closely at the next picture. Study the graph and then look at Arden's little face in the shadows. Imagine all of the people living with diabetes that are tired, battling a cold or just would love, absolutely f&%king love, a decent nights rest and then do something to help them.

I know what the FDA will say, they may say that if the signal is carried by my home WiFi for example then my home WiFi would need to be verified so it's not possble. They may say any number of a thousand things, but I don't care. Tell me where to sign my rights away. I promise I won't sue anyone if my router happens to stop working on the night my daughter's BG gets so low that it harms her. I'll take the chance, it's mine to take. Stop restricting great ideas from seeing the light of day because of what might go wrong. Something is better then nothing. Right?
Seat belts aren't 100% effective, I still wear one. Condoms can't promise we won't get sick or pregnant, but we wear them because it gives us a better chance. This isn't just an issue for young children and their parents. This is also about husbands and wives, boyfriends, college kids, it's an issue that effects every person with diabetes and all we want is a fighting chance.
Medtronic found a way around the problem with MySentry. Sanofi figure out how to link an app with iBGStar. It can be done.
Here is my unsolicited message to every company that has an idea that they are afriad to move forward with because of government regulations: I know you are scared to approach the FDA with innovation, I get it, I really do understand. But I'm afraid to sleep. I'm afriad that my daughter is going to die. People are scared that their wives, friends, mothers, sons will fall to sleep one night and never wake up. We win, our fear is worse then yours. Help us.
 Friday, August 30, 2013 at 2:01PM
Friday, August 30, 2013 at 2:01PM  Advocacy,
Advocacy,  DOC,
DOC,  FDA,
FDA,  Petition,
Petition,  diabetes in
diabetes in  Daddy's Blog,
Daddy's Blog,  Type I News
Type I News